β-glucans: Immunomodulatory effects & Mortality control
Melina Bonato (Coordinadora de I&D, ICC Brasil)
For a long time, the most common method for dealing with the occurrence of bacterial infections in aquaculture was the administration of antibiotics. However, aquaculture faces serious problems due to various adverse effects of these drugs such as accumulation in the tissue and environmental microbial flora. Moreover, to use antibiotic or vaccine for fish is expensive and in many farms its use is unavailable (Yousefian and Amiri, 2009). Thus, the use of substances or additives incorporated into the feed to improve the survival rate, disease resistance and growth of fish and shrimp has been used more frequently and more successfully.
Fish, similar to mammals, have the innate and the adaptive immune systems, with the innate being responsible for primary responses, where the response is fast, is not specific, and with no memory against recontaminations, and the adaptive is the responsible for specific responses, in other words, intense response with specific antibodies for each pathogen.
The most widely known cells of the innate immune system are the macrophages, neutrophils, dendritic cells and natural killer cells (Sharma, 2003). Toll-like receptors, located on the surface of immunological cells, recognize microbial patterns and induce an immediate innate immune response. After this activation and phagocytosis, the phagocyte presents a processed fragment of the pathogen to the adaptive immune system and stimulates an anti-pathogen response. Therefore the phagocytes are called antigen-presenting cells. The recognition of pathogens by the innate immune system triggers immediate innate defenses and activation of the adaptive immune response (Lee & Iwasaki, 2007).
ImmunoWall® from ICC Brazil is a product composed by the cell wall of the Saccharomyces cerevisiae yeast, originating from sugarcane fermentation process for ethanol production. This product contains around 35% of β-glucans and 20% of mannan oligosaccharides (MOS). β-glucans are known as immune system modulators or stimulants, because when they come in contact with the phagocytes, which recognize the β-1,3 and 1,6 bindings (Petravic-Tominac et al., 2010), these are stimulated and will produce some cytokines, which will start a chain reaction inducing an immunomodulation and improving the response capacity of the innate immune system. (Figure 1).
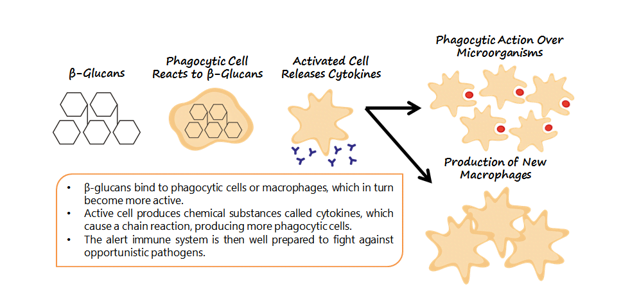
Figure 1. Macrophages inhabit the intestinal mucosa. When they come into contact with the 1-3 and 1-6 ß-Glucans found in yeast cell walls, the interaction triggers activation of the immune system.
This type of response is especially important in animals in initial growth, reproductive phases, stress periods, and environmental challenges; acting as a prophylactic agent and increasing animal resistance, minimizing further damages (such as a drop in performance or high mortality rates). Intensive animal production is a highly challenging environment. Thus the strengthening of the immunological system can be one of the keys toward greater productivity.
MOS is known for their pathogen agglutination capacity (mainly those with type 1 fimbria), as well as, diverse gram-negative strains. MOS offers a binding site for pathogens, preventing the colonization of the intestinal epithelium, and these agglutinated bacteria will be excreted together with the indigestible part of the fiber (Figure 2).
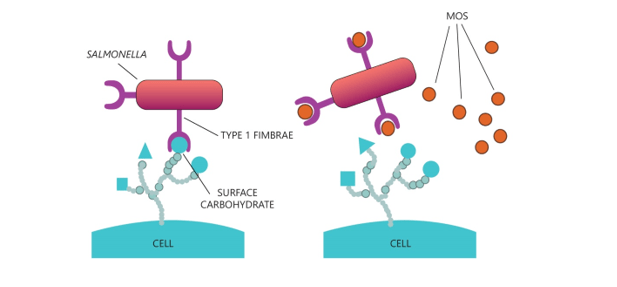
Figure 2. Scheme of pathogens with type I fimbriae adhering to the cells of the intestine (left side), and in the presence of MOS (right side).
In a recently study conducted at Faculty of Veterinary Medicine, Cairo University, Egypt by Abu-Elala, et al. (data not published), 270 Oreochromis niloticus (50.7±0.8 g of BW) were divided into 3 experimental groups: Control , 0.1% and 0.2% of ImmunoWall®; being 90 fishes for each treatment (3 replicate/tanks). During 2 months the performance of fishes was measured at every 2 weeks, and at the end of the trial, 5 fishes/replicate were euthanized in order to evaluate clinicopathological, oxidant and antioxidant parameters, relative quantitative PCR of immune gene expression, phagocytic activity (%) and index, lysozyme activity (µg/mL). After 2 months, the fishes were challenged against gram-positive bacteria Lactococcus gravaeie, and gram-negative Aeromonas hydrophila and the mortality rates were observed during 1 week.
At two months 0.2% ImmunoWall® improved 25.9 g body weight than the control group. ImmunoWall® supplementation is reaching an increase of 46.36% to weight gain and reduced 21% to feed conversion ratio concerning the control group (P>0.05).
ImmunoWall® improved clinicopathological results (WBCs, TP, and Globulin) as well as, relative quantitative PCR expression of IL1-β and phagocytic and lysozyme activities (P<0.05). Dietary supplementation of ImmunoWall® increased catalase and g-reductase activities (P<0.05). After the challenge with Lactococcus gravaeie and Aeromonas hydrophila, mortality decreased (P<0.05) in both groups with ImmunoWall® supplementation (Table 1).
Table 1. Performance, clinicopathological results, oxidative stress, expression of immune-related genes, innate immunity and mortality parameters of O. niloticus
| Parameters | Control | 0.1% ImmunoWall® | 0.2% ImmunoWall® |
| BW (g) 2 months | 94.86b | 118.5a | 120.8a |
| BWG (g) | 48.1b | 67.8a | 70.4a |
| FCR | 2.1b | 1.73a | 1.66a |
| WBCs | 130b ± 13.02 | 201a ± 20.13 | 156b ± 15.62 |
| TP | 2.23b ± 0.25 | 2.97a ± 0.23 | 2.27b ± 0.27 |
| Globulin | 1.21b ± 0.12 | 1.94a ± 0.13 | 1.16b ± 0.12 |
| Catalase | 268.73b ± 25.39 | 588.73a ± 42.05 | 618.3a± 60.7 |
| G-redutase | 142.70c ± 3.55 | 192.93b ± 21.25 | 269.27ª ± 20.61 |
| IL1-β | 0b | 7.5b ± 1.1 | 16a ± 1.3 |
| Phagocytic activity (%) | 57b | 61a | 70ª |
| Phagocytic index | 1.8b | 1.75b | 2.5ª |
| Lysozyme activity (µg/mL) | 435.8b | 450.95a | 464.3ª |
| Mortality (%) L. gravaeie | 90b | 50a | 60ª |
| Mortality (%) A. hydrophila | 100b | 40a | 50a |
abMeans with different letters in the same row differ significantly by Tukey test (P<0.05). BW: body weight. BWG: body weight gain. FCR: feed conversion rate. WBCs: white blood cells. TP: total protein.
In conclusion, the inclusions of 0.1 and 0.2% of ImmunoWall® were able to improve clinicopathological response and innate immunity. ImmunoWall® supplementation increased oxidative enzymes activity and reduced mortality rates when the fishes were challenged with Lactococcus gravaeie and Aeromonas hydrophila, compared to the control group. The decrease in mortality rates after the challenges was consistent and represented at least 50% more survival rates in cases where there is 100% mortality due to pathogen contamination.
Other studies have been published about benefits of yeast cell wall supplementation in aquaculture. Ebrahimi et al. (2011) studying common carp fingerlings (Cyprinus carpio) infected with Aeromonas hydrophila found a significant increase in survival rates number of leucocytes and improvement in feed conversion ratio results in groups fed diets with ImmunoWall® from 1 to 2.5%. Ebrahimi (2010) reported a decrease in total bacteria counts in the intestine and an increase in survival rate and feed conversion ratio improvement of Rutilus frisii Kutum fingerlings fed with ImmunoWall® from 0.5 to 2.5%. Karimzadeh et al. (2013) studying Rutilus kutum larvae found an improvement in survival rate, final body weight, and feed conversion ratio, as well as a decrease in total bacteria counts in the intestine at 0.5% of ImmunoWall® supplementation.
Enhance and modulate the innate immune system may be one of the strategies to combat contamination, reducing mortality and improve productivity. If dietary yeast cell wall is supplied early to the animals, the immune system will be modulated and will be alert to many infections or contaminations. The action of the β-glucan occurs on the innate immune system, in other words, where the first immune response to pathogen contamination happens, avoiding a greater expenditure of energy during a prolonged inflammation process and mobilizing the adaptive immune system faster, avoiding losses in production and high mortality rates.
References
Abu-Elala, N. M., et al. (2018) Dietary influence of Immunowall® on the growth performance, haematological\oxidative stress parameters and immunity of cultured Oreochromis niloticus (data not published).
Ebrahimi, G. (2010) Effects of prebiotic supplementation on survival, growth performance and feed utilization of Kutum, Rutilus frisii (Kamenskii), fingerlings. Research Journal of Animal Science, 4 (6): 125-129.
Ebrahimi, G. et al. (2012) Effects of a prebiotic, Immunogen®, on feed utilization, body composition, immunity and resistance to Aeromonas hydrophila infection in the common carp Cyprinus carpio (Linnaeus) fingerlings. Journal of Animal Physiology and Animal Nutrition, 96: 591–599.
Karimzadeh, S. et al. (2013) Effects of Different Levels of Immunogen® on Growth Performance, Intestinal Bacteria Colonization and Survival Rate in Rutilus kutum Larvae. World Journal of Fish and Marine Sciences 5 (6): 664-669.
Lee, H. K., A. (2007) Iwasaki. Innate control of adaptive immunity: dendritic cells and beyond. Semin. Immunol., n. 19, p.48-55.
Lee, H. K. and A. Iwasaki (2007). Innate control of adaptive immunity: dendritic cells and beyond. Semin. Immunol., n. 19, p.48-55.
Petravić-Tominac, V. et al. (2010). Biological effects of yeast β-glucans. Agriculturae Conspectus Scientificus, n. 75, v. 4.
Sharma, J. M. (2003). The avian immune system. In: Saif, Y. M. (ed.), Diseases of Poultry, 11th ed., Iowa State Press, Ames, IA, pp. 5-16.
Yousefian, M. and Amiri, M.S. (2009). A review of the use of prebiotic in aquaculture for fish and shrimp. African Journal of Biotechnology. Vol. 8 (25), pp. 7313-7318.
*Author information: Melina Bonato is Ph.D. in Animal Science. Since 2013 she has been coordinating the research and development department at the ICC Brazil, which is focused on yeast-based products studying animal nutrition, immune responses, health, and performance.
Posted in 25 June of 2019


